Preparing for implementation of new TB vaccines:

Preparing for implementation of new TB vaccines
SATVI researchers participate in high level discussions to ready South Africa for new TB vaccines

SATVI Professor Mark Hatherill and Associate Professor Angelique Kany Kany Luabeya, from the South African Tuberculosis Vaccine Initiative (SATVI), based at UCT, recently participated in the first TB vaccine preparedness workshop convened to develop a country-level roadmap for the introduction of new TB vaccines which be might approved following several late-phase TB vaccine trials being conducted in South Africa.
Preparing for vaccines
The engagement was convened by the South African National Department of Health and the World Health Organisation, with the aim to take stock of the current investigational TB vaccine pipeline; and to map the complex task to implement a new TB vaccine roll-out for adolescents and adults. The engagement considered the current TB vaccine pipeline, what is need to strengthen vaccine supply chain readiness, financing such new vaccines, community engagement, health worker training and policy frameworks for the introduction should these a new TB vaccine candidate be approved.

The burden of disease
Tuberculosis disease, caused by Mycobacterium tuberculosis (M.tb), is a major public health problem in South Africa and the leading cause of death amongst people living with HIV. In 2023, 270 000 people fell ill with TB in South Africa ; 56 000 people died from TB, of whom 31 000 were people living with HIV.
“These are the very groups most at risk of getting sick and transmitting TB.”
Dr Aaron Motsoaledi, Minister of Health
South Africa is one of 30 countries that account for 87% of estimated TB cases, globally. Despite very good coverage of bacille Calmette-Guérin (BCG) vaccination at birth, BCG of infants offers limited protection against adult forms of TB responsible for transmission, since its protective effect wanes before adolescence.
Setting the scene for new TB vaccines
The workshop, which brought together health policymakers, vaccine developers, funders, leading TB researchers and community leaders, considered various elements of a national strategy and the gaps in research, resources and current capacity, which would need to be addressed in a coordinated manner to prepare for implementation of new TB vaccines.
“With several new TB vaccine candidates in the late stages of clinical trials, the most promising options are expected to be available in the next few years.”
Strategic deployment of new TB vaccines to attain optimal impact on burden of disease
Professor Mark Hatherill, SATVI Director, spoke on TB prevalence and risk among various priority populations who could potentially benefit from early implementation of a new effective TB vaccine, in terms of feasibility, equity and impact.
According to Professor Hatherill the optimal impact of new TB vaccines might be in the following groups:
- People between the ages 15 and 44, who are at high risk of TB, characterised by high rates of M.tb sensitisation (upwards of 50%), in geographical areas with high TB rates. This age group is marked by a rapid rise in TB infection and disease rates amongst adolescents.
- Health workers, who due to their occupational settings are exposed to drug-sensitive and drug-resistant TB, and have an elevated risk of TB disease. Health workers also provide healthcare for others, including in the TB control programme.
- People Living with HIV, who are at a greater risk of getting TB and dying from TB. However, the lack of efficacy data for this group from current Phase 2b and 3 trials means that the impact of TB vaccination on prevention of HIV-associated TB might only be known following downstream post-licensure studies.
Overview and prospective TB vaccine candidates
Associate Professor Angelique Luabeya discussed the candidate TB vaccine pipeline and ongoing TB vaccine research being conducted in South Africa.
Modelling suggests a new TB vaccine with only 50% efficacy for adolescents/adults might prevent 37 million TB cases and 4.6 million deaths worldwide, between the years 2025 and 2050. It would be cost-effective in all high TB burden countries and bring about cost-savings in 58 of 105 (55%) LMIC.
A new TB vaccine with only 50% efficacy for adolescents/adults might prevent 37 million TB cases and 4.6 million deaths worldwide, between the years 2025 and 2050. It would be most cost-effective in all high TB burden countries and bring about cost-savings in 58 of 105 (55%) LMIC. Angelique Luabeya, SATVI
A/Prof Luabeya stressed that South Africa has emerged as a leader in research into new TB vaccines, diagnostic tools and therapies, as well as TB co-morbidities like HIV and diabetes. A/Prof Luabeya has led clinical trials of several TB vaccines including H56:IC41, ID93 + GLA/SE, VPM1002, BCG and MTBVAC, in adult, adolescent, and infant populations at SATVI. The University of Cape and other South African research groups, including The Aurum Institute, Stellenbosch University, WITS RHI, AHRI and CAPRISA have made a substantial impact on the global TB vaccine research agenda.
South Africa, a prime setting for researching TB
South African has become a key setting for TB research because of the high TB incidence, high rates of TB/HIV co-morbidity, and the fact that universal infant BCG vaccination has enabled studies of the booster effects of new vaccines. TB vaccine research conducted in South Africa will mean that the findings of ongoing efficacy trials are directly relevant to the South African TB epidemic.
New TB vaccine candidates in clinical testing
A/Prof Luabeya, presented various TB vaccine candidates, which include: -
- M72/AS01E Phase 3 trial: Efficacy of this vaccine is being studied in seven countries, including South Africa, in adolescents and adults (15-45) living in TB endemic areas. 60% of the study population (n=20,000) enrolled are in South Africa.
- MTBVAC Phase 2/b trial: This efficacy trial of MTBVAC, funded by the International AIDS Vaccine Initiative (IAVI) is being conducted at 15 clinical research sites, including 4 South African sites, as well as in Kenya and Tanzania. The primary study population are adolescents and adults (14-45) living in TB endemic areas.
- MTBVAC Phase 3 trial: This efficacy trial of MTBVAC, funded by EDCTP and Biofabri, is being conducted in healthy, HIV-unexposed and HIV-exposed, -uninfected infants, in Madagascar, Senegal and six sites in South Africa.
- A Phase 1 trial of BNT164, the first mRNA TB vaccine presenting M.tb antigens delivered via lipid nanoparticles to enter clinical development, is being conducted at SATVI, UCT Lung Institute, AHRI and CAPRISA.
- H107e/CAF10b, a subunit vaccine which combines H107e and CAF®10b, is being studied in adults with and without M.tb infection at two sites in South Africa.
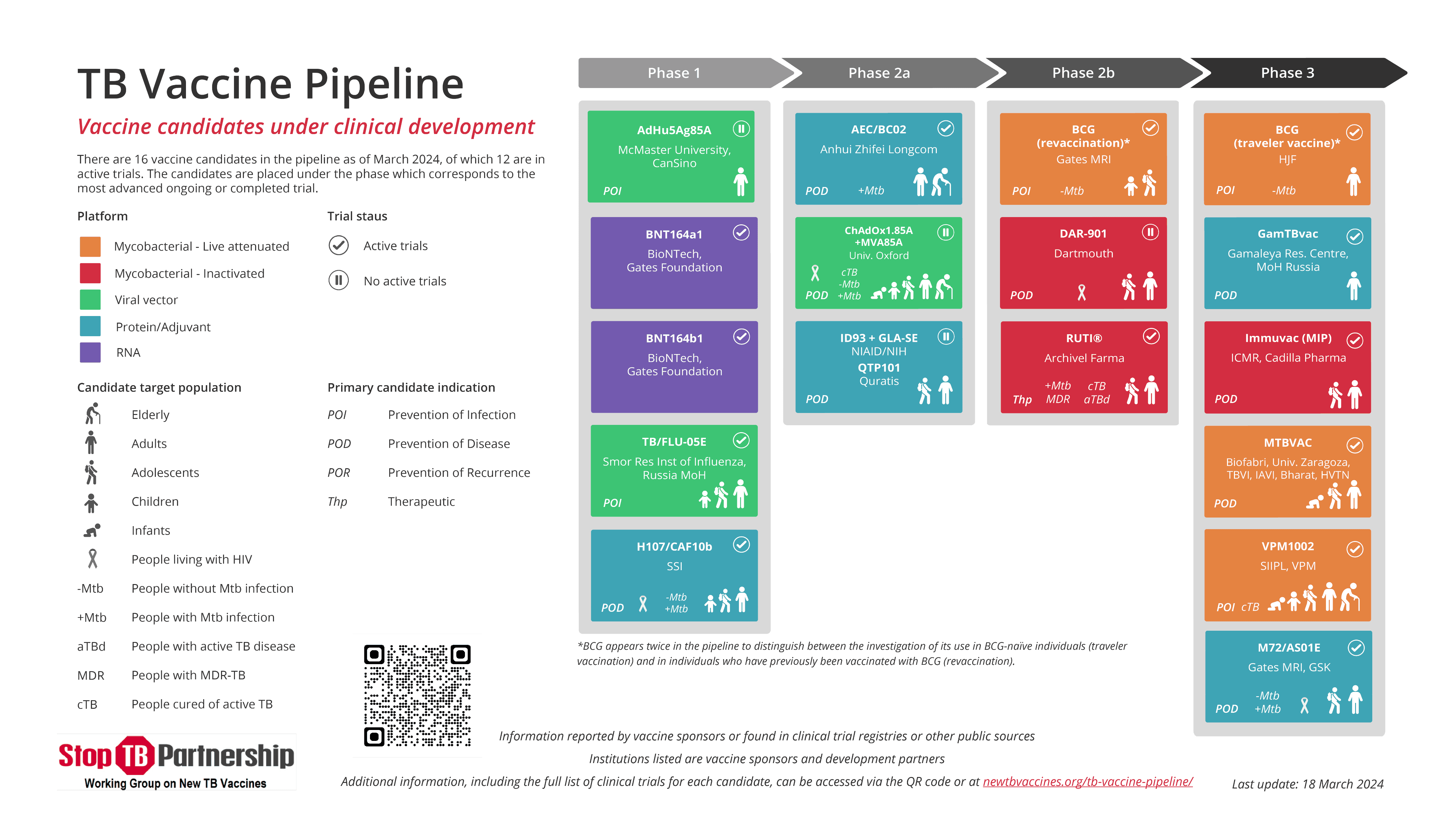
Source: Stop TB Partnership, Working Group on new Vaccines
Addressing the unknowns
A/Prof Luabeya also highlighted several unknown areas of TB vaccine research, which include vaccine efficacy in high-risk populations, duration of protection, and optimal delivery strategies.
Strengthening South Africa’s role in global TB vaccine development
South Africa’s central role in global TB vaccine development lies in leveraging the expertise of leading investigators in this field; expanding existing trial sites to include rural areas to improve generalisability of data; strengthening local manufacturing & innovation through investment, for example in mRNA Technology through Afrigen Biologics, a WHO-designated mRNA hub; learning vaccine advocacy lessons from the COVID-19 pandemic to strengthen vaccine confidence; involving SA communities in TB vaccine trials to allow SA priorities to be part of the TB vaccine development agenda; and lastly making use of existing SA regulatory expertise to accelerate vaccine licensure.
END
