Research: Shortened 4-month TB treatment offers breakthrough in TB control.

The New England Journal of Medicine has published the results of a clinical study which shows that in a randomised phase 3 clinical trial, a shortened 4-month TB treatment was equivalent to the standard 6-month TB treatment.
The study involved 2 154 participants between with newly diagnosed drug susceptible pulmonary TB in three groups. The first group, the control group (Arm 1) received the standard 6-month treatment consisting of rifampicin, isoniazid pyrazinamide and ethambutol. In the other two groups, (Arm 2) one received a 4-month regimen with rifampicin replaced with rifapentine and in the other group (Arm 3), rifampicin was replaced with rifapentine and ethambutol replaced with moxifloxacin. The study included people living with HIV, which was controlled.
“These results are a game-changer for shortening treatment of TB patients in developing countries.”
Professor Mark Hatherill SATVI Director,
This phase 3 randomised clinical trial was conducted as part of an international, multicentre trial within the International Trials Consortium Study 31/AIDS Clinical Trials Group (ACTG) and funded by the National Institutes of Health (NIH).
These are exciting findings which could have a massive impact on how we manage patients with pulmonary TB. A 33% reduction in the duration of TB treatment could improve adherence and reduce the cost associated with treatment of TB patients. Dr Justin Shenje, SATVI Investigator
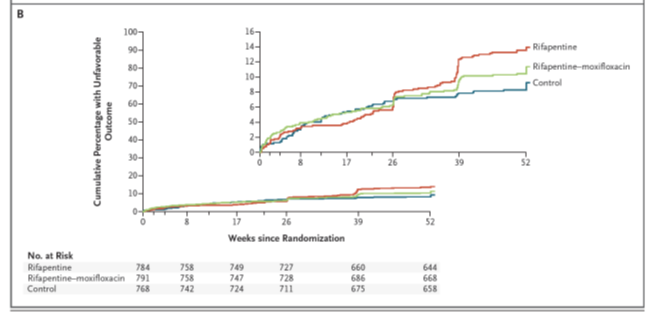
Figure: Primary and secondary efficacy analysis
Extra resources
The article is accompanied by a supplement, editorial, video and Research Summary.
