Research: Immune serum-activated human macrophages coordinate with eosinophils to immobilize Ascaris suum larvae

Professor Mark Hatherill has co-authored a research paper titled "Immune serum-activated human macrophages coordinate with eosinophils to immobilize Ascaris suum larvae" appearing in the Parasite Immunology Journal.
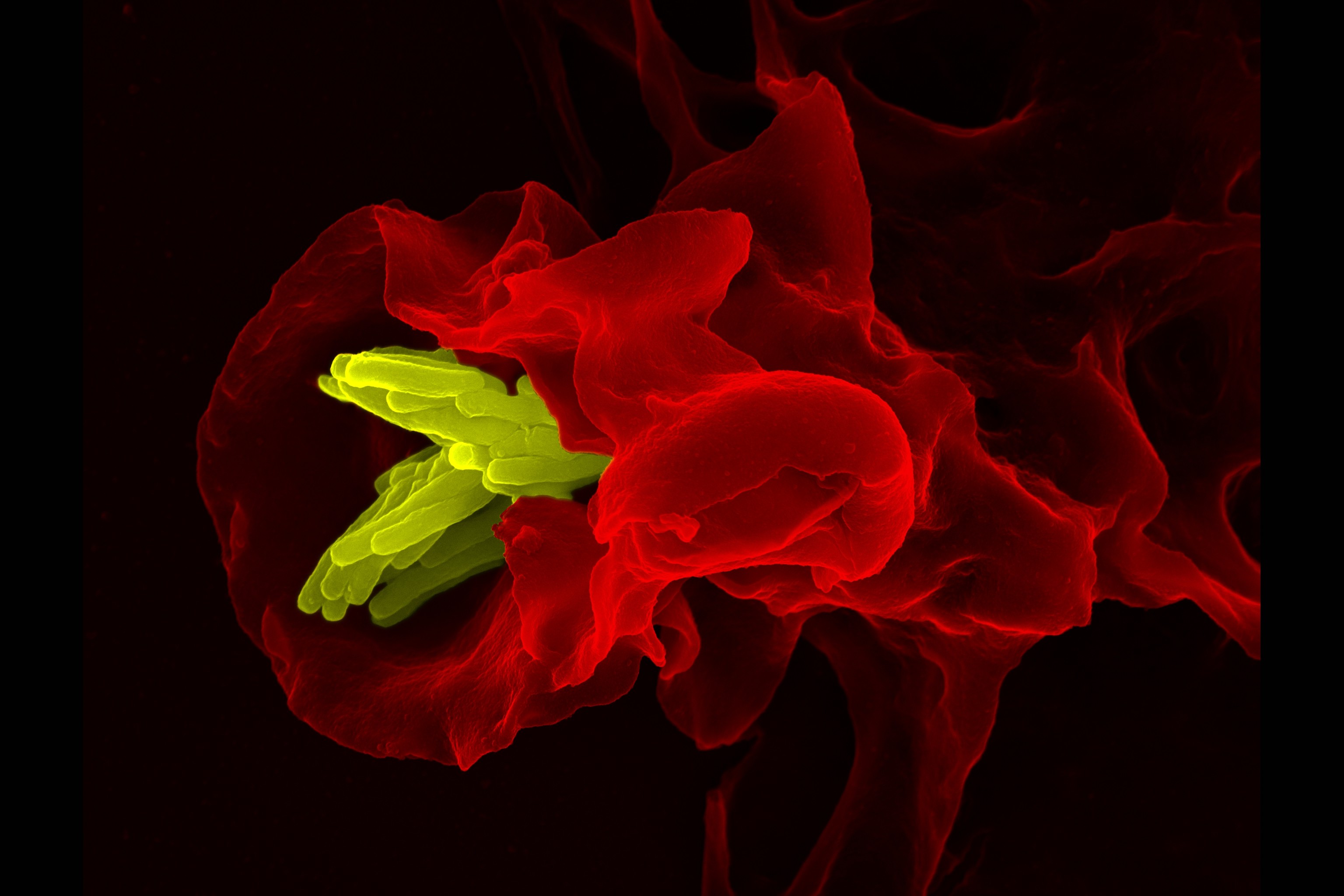
Background: Helminth infection represents a major health problem causing approximately 5 million disability-adjusted life years worldwide. Concerns that repeated anti-helminthic treatment may lead to drug resistance render it important that vaccines are developed but will require increased understanding of the immune-mediated cellular and antibody responses to helminth infection. IL-4 or antibody-activated murine macrophages are known to immobilize parasitic nematode larvae, but few studies have addressed whether this is translatable to human macrophages. In the current study, we investigated the capacity of human macrophages to recognize and attack larval stages of Ascaris suum, a natural porcine parasite that is genetically similar to the human helminth Ascaris lumbricoides. Human macrophages were able to adhere to and trap A suum larvae in the presence of either human or pig serum containing Ascaris-specific antibodies and other factors. Gene expression analysis of serum-activated macrophages revealed that CCL24, a potent eosinophil attractant, was the most upregulated gene following culture with A suum larvae in vitro, and human eosinophils displayed even greater ability to adhere to, and trap, A suum larvae. These data suggest that immune serum–activated macrophages can recruit eosinophils to the site of infection, where they act in concert to immobilize tissue-migrating Ascaris larvae.
Discussion: The current study aimed to understand the role of human macrophages in mediating protective immunity against nematode parasites. We observed that human blood monocyte-derived macrophages could adhere to the surface of A suum L3 and impair their motility in response to activation by immune serum. Of special interest, human macrophages activated by immune serum and A suum L3 upregulated expression of the eosinophil chemokine CCL24. And eosinophils were observed to be significantly better than macrophages at trapping larvae. Tissue-resident macrophages are likely to be some of the first cells able to respond to invading larvae, and our data indicate that in immune individuals, these cells may serve a dual purpose in mediating protective immunity. In the first instance, they would recognize and adhere to the invading larvae, perhaps also impacting negatively on larval migration. Next, they would secrete CCL24 prompting eosinophils to exit the circulation and migrate to the site of larval infection. At this point, macrophages and eosinophils would co-operate to immobilize, and eventually kill, the larvae interrupting larval migration and developing and halting the infectious cycle. Through the course of natural infection, L3 are found in the intestine of both Ascaris-infected humans and pigs.14, 22 Importantly, immunized A suum-infected pigs exhibited a strong eosinophilic response in the intestinal mucosa, limiting larval migration to the liver and lung.22
Although this model remains hypothetical, it is in keeping with histological studies from a wide range to host and parasite species indicating the presence of both macrophages and eosinophils in close proximity to tissue-migrating helminth larvae. It also coincides with findings in murine H polygyrus infection, whereby challenge-infection of mice leads to the rapid accumulation of Arginase-1 expressing macrophages around larvae in the intestinal serosa, with a later influx of eosinophils.41 It is also supported by a recent study in Brugia malayi-infected mice, in which macrophages mediated immunity via recruitment of eosinophils to the peritoneal cavity in a CCR3-dependent fashion.63 An increase in circulating CCL24 levels has also been reported in Schistosoma mansoni patients during acute infection and in patients given anti-helminthic treatment against Oncocerca volvulus.64, 65 Furthermore, CCL24 production was highly elevated in peripheral blood mononuclear cells (PBMCs) isolated from Ascaris-infected adults following exposure to A lumbricoides antigen extract.66 Taken together, these data indicate that macrophages may often function to produce eosinophil chemoattractants after encountering parasitic larvae and that both cell types may act in concert to trap and/or kill the larvae.
Whilst Ascaris infection is classically associated with increases in both total and specific IgE, other antibody isotypes are also shown to be elevated.13 Indeed, the concept of isotypic selection and balance may contribute to resistance, as serum IgG4 levels were shown to inversely correlate to worm burdens in Ascaris infection,18 clinically symptomatic filariasis and onchocerciasis67 and in patient cohorts treated with a recombinant vaccine against Schistosoma japonicum.68 As such, the identification and selection of both specific antibody isotypes and candidate antigens represent an essential step in successful vaccine design. To this end, it was recently shown that mice vaccinated with crude A suum antigens rely on IgG1 and IgG3 subclasses for protection,69 and vaccination against a 16-kDa protein expressed in both human and pig Ascaris was found to confer protection in experimental models of infection by inhibiting larval migration.70, 71 Interestingly, naïve serum was not sufficient to induce any larval recognition or trapping by human macrophages or eosinophils, suggesting that complement alone does not mediate this response. This was intriguing given a previous study that demonstrated the ability of porcine eosinophils to degranulate and kill A suum larvae in the presence of naïve serum, suggesting a mechanism which is partially complement-dependent.38 These differences may highlight some fundamental distinctions in immune-effector mechanisms between host species during Ascaris infection. From our observations, it is yet unclear as to which components present in immune serum may activate human macrophages and eosinophils, with further work necessary to prove a specific role for complement, particular antibody isotypes, cytokines and/or other molecules which may contribute to this response. Whilst the addition of IL-4 to our serum co-cultures did not enhance binding of cells to larvae in vitro, previous studies have demonstrated that both IL-4 and IL-10 drive expression of CCL24 in both human monocyte-derived macrophages and murine bone marrow macrophages72, 73 and may suggest a role for these cytokines in driving a subsequent immune cascade through recruitment of eosinophils.
Discussion: In summary, we have shown that human macrophages are able to adhere to, and trap, A suum larvae following their activation by immune serum. We have also shown that immune serum–activated macrophages secrete chemokines to attract eosinophils and that immune serum–activated eosinophils exhibit an even more potent capacity for larval trapping. As underlying mechanisms mediating macrophage trapping of larvae differed from that reported for rodent models of helminth infection, further studies will be required to understand the precise mechanisms by which these cells trap Ascaris larvae, and to determine the possible contribution of cell types other than macrophages and eosinophils, as well as a combination of other mediators including cytokines and complement. Determining the antibody isotypes and other factors involved in eliciting macrophage and/or eosinophil-mediated protection will also be informative as this could provide important information for the design of much needed protective vaccines against these insidious parasites.
