Research: Determining M. tuberculosis components to be included in vaccines

Researchers from SATVI have collaborated with the Center for Infectious Disease Research (CIDR), Statens Serum Institut (SSI) publishing a paper providing important insights for determining which components of Mycobacterium tuberculosis should be included in TB vaccines as targets for the immune response.
The study provides important insights for determining which components of Mycobacterium tuberculosis should be included in TB vaccines as targets for the immune response. The researchers studied T cells, white blood immune cells that are critical for immunity against M. tuberculosis, that recognise two important proteins made by M. tuberculosis, ESAT-6 and Ag85B. These two proteins are included as immune response targets, or antigens, in a number of TB vaccine candidates that are currently being tested.
The researchers studied T cells, white blood immune cells that are critical for immunity against M. tuberculosis, that recognise two important proteins made by M. tuberculosis, ESAT-6 and Ag85B. These two proteins are included as immune response targets, or antigens, in a number of TB vaccine candidates that are currently being tested.
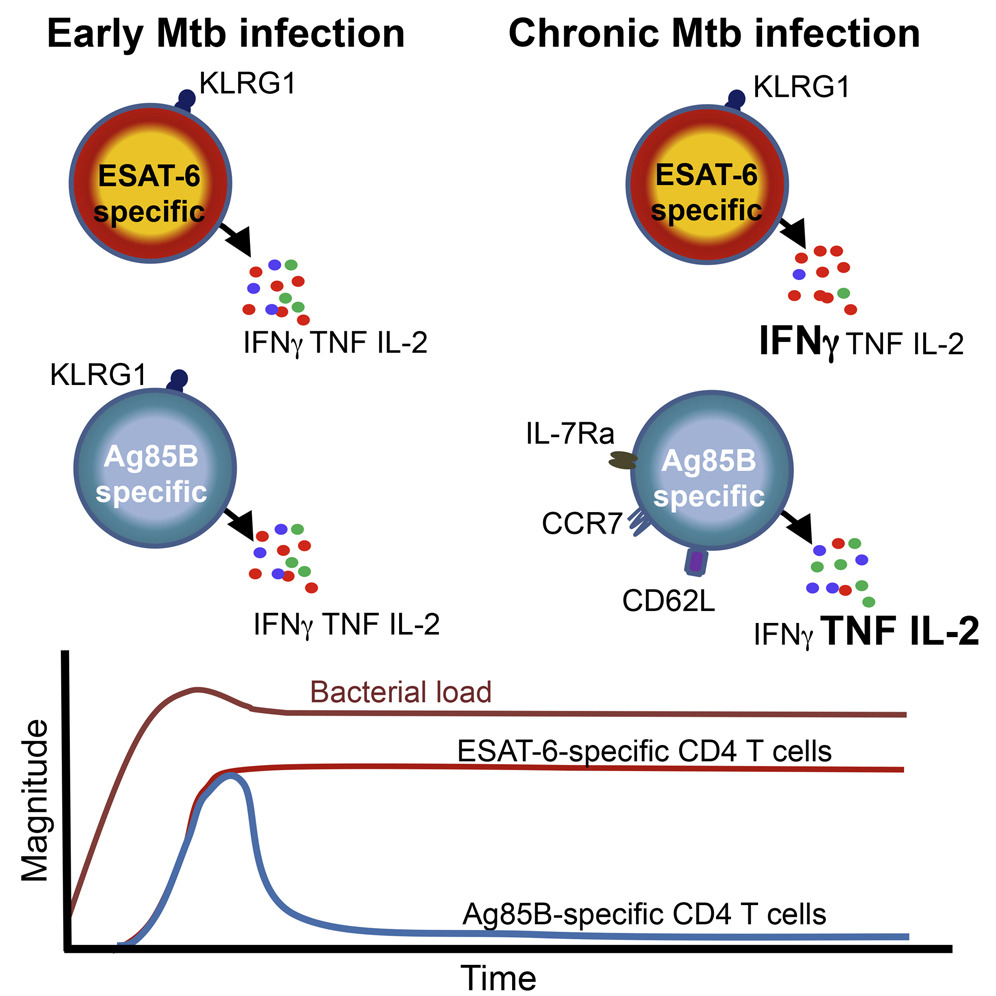
Although both antigens were recognised by T cells during M. tuberculosis infection of mice and humans, the T cells that recognised Ag85B were very different in function from those that recognised ESAT-6. Ag85B and ESAT-6-specific cells were also limited in their ability to do their job, namely to restrict growth of the M. tuberculosis bacterium, but for opposite reasons. Ag85B-specific T cells did not find sufficient Ag85B protein to respond to, likely because the bacterium makes only very small amounts of this protein, and therefore could not recognise cells infected with M. tuberculosis. By contrast, ESAT-6-specific cells were exposed to too much ESAT-6 protein, produced in vast amounts by the bacterium in a continuous manner throughout infection. As a consequence, much like a marathon runner who cannot sprint the entire distance, the T cells that respond to ESAT-6 become exhausted, lose function and cannot effectively fight the bacterium.
These findings pave the way for assessment of strategies to overcome these limitations in the T cell response and to assess other antigens for inclusion as immune targets in TB vaccines
Discussion:
Our data have important implications for the rational design of TB vaccines tailored to optimize protection conferred by specific CD4 T cells recognizing antigens expressed at distinct stages of Mtb infection. We report definitive evidence that some antigens, like Ag85A and Ag85B, are expressed almost exclusively during early infection, whereas other antigens, like ESAT-6, are expressed throughout infection. Although prior Mtb transcript profiles from lungs of Mtb-infected mice have suggested this (Rogerson et al., 2006,Shi et al., 2004), it has not gained widespread acceptance, perhaps in part because the relevance to human Mtb infection has not been clear. Our studies in mice showed that CD4 T cells recognizing persistently expressed antigens accumulate in high numbers in the lung parenchyma and are driven toward terminal differentiation/functional exhaustion that compromises their protective capacity. In contrast, T cells recognizing antigens expressed primarily during early infection contract in numbers, assume a less differentiated phenotype akin to memory cells, and retain the capacity to produce multiple cytokines. The finding that human CD4 T cells recognizing Ag85A and Ag85B are less differentiated than those recognizing ESAT-6 strongly suggests that Ag85A and B exhibit low levels of expression during chronic stages of human Mtb infection, as in mice, whereas ESAT-6 is expressed persistently in both species.
How do these findings shape the protective capacities of the T cells that recognize them? The murine data suggest that Ag85B- and ESAT-6-specific CD4 T cells may contribute to Mtb control. However, the protection provided by each appears sub-optimal, but for different reasons. As shown previously (Bold et al., 2011), serial infusions of Ag85B peptide can reduce the lung bacterial burden. There are two possible explanations for this: (1) T cells recognize the infused peptide directly presented by MHCII molecules on the surface of Mtb-infected cells in the lung, facilitating a cognate interaction that does not otherwise occur without peptide infusion, and/or (2) T cells recognize the infused peptide on uninfected cells and the bolus of cytokine produced activates infected cells and promotes some Mtb killing in a bystander manner. We now show that while this is true for the Ag85B peptide, infusions of ESAT-6 peptide do not reduce bacterial numbers. Thus, ongoing recognition of infection-derived ESAT-6 antigen is probably stimulating ESAT-6-specific T cells to their full capacity to eradicate Mtb. The observation that Ag85B peptide infusion, which promotes the activation of T cells with robust capacity to produce cytokines, can improve clearance despite ongoing recognition of ESAT-6 suggests that this “full capacity” of ESAT-6-specific T cells is still sub-optimal. In other words, protective immunity conferred by Ag85B-specific T cells is limited by antigen availability during chronic infection, whereas immunity by ESAT-6-specific T cells is limited by functional exhaustion. The role of antigen availability in shaping the differentiation, function, and protective capacity of Mtb-specific CD8 T cells needs to be addressed in future studies.
Understanding the distinct limitations of specific T cell subsets suggests unique vaccination strategies to enhance the protection that each confers. It is likely that vaccines based solely on antigens like Ag85B, which are primarily expressed in early infection, would confer only transient protection and may possess a limited capacity to promote Mtb containment after infection has been established. These problems may be exacerbated with systemically administered vaccines because peripheral T cells are delayed in their recruitment to the lungs after aerosol Mtb infection (Urdahl, 2014). Could reduced expression of Ag85A during chronic stages of Mtb infection underlie the lack of protection afforded by the MVA85A vaccine in two recent Phase IIb trials (Ndiaye et al., 2015, Tameris et al., 2013)? Antigens like Ag85B may yet be useful in vaccines that induce lung-resident T cells that confer early Mtb control after aerosol infection, especially if used in conjunction with late-stage antigens, like ESAT-6, that can act in concert to promote containment once infection is established.
Our findings help explain why vaccines containing ESAT-6, but not several other antigens, including Ag85B, were capable of conferring protection when administered to mice after Mtb infection had been established (Hoang et al., 2013). Yet our studies in mice also suggest that functional exhaustion can restrict the protective capacity of CD4 T cells recognizing persistently expressed antigens. While recent murine studies suggest that lung resident T cells can become functionally exhausted (Behar et al., 2014, Jayaraman et al., 2016), our study shows that T cells recognizing distinct Mtb antigens are differentially prone to this. Although functional exhaustion was not observed in ESAT-6-specific T cells isolated from the blood of Mtb-infected humans, this could reflect differences between the blood and lung, or between latent and active TB, rather than differences between species. Two recent murine studies have shown that Mtb-specific T cells in the blood have a higher cytokine-producing capacity than those in the lung parenchyma (Moguche et al., 2015, Sakai et al., 2014a). Likewise, in cynomologous macaques, ESAT-6-specific T cells in the blood were capable of producing multiple cytokines, but T cells isolated from lung granulomas produced multiple cytokines only after polyclonal stimulation, but not after stimulation with ESAT-6 peptides (Gideon et al., 2015). Furthermore, in rhesus macaques, LAG3, a cell-surface inhibitory receptor associated with CD4 T cell exhaustion in this species (Bosinger et al., 2009, Chew et al., 2016, Fromentin et al., 2016, Rotger et al., 2011), was expressed during TB by lung T cells, but not the blood (Phillips et al., 2015). Thus, antigen-specific T cells may have greater functional capacity in the blood than at the site of high antigenic exposure in the lung. This is even more likely to be true during active TB disease when bacterial burdens are highest.
The challenge of devising a T cell-targeted vaccine against Mtb is heightened by the recent understanding that antigen-specific memory CD4 T cells that are less differentiated exert greater control of murine Mtb infection than highly differentiated effector cells (Lindenstrøm et al., 2013 , Moguche et al., 2015, Sakai et al., 2014a, Torrado et al., 2015, Vogelzang et al., 2014
). The protective capacity of these “less differentiated” CD4 T cells reflects their superior ability to proliferate, maintain their cytokine-producing capacity, and home to infected tissues (Andersen and Urdahl, 2015, Sakai et al., 2014b; Woodworth et al., 2017
). Thus, while choosing persistently expressed vaccine antigens is likely to be important, these same antigens may drive T cells toward terminal differentiation and dysfunction. These considerations must be heeded in choosing the adjuvants and the antigen doses utilized in the next generation of TB vaccines. In some regions endemic for TB, where an effective TB vaccine is most needed, most individuals have already acquired Mtb infection by late adolescence (Andrews et al., 2014 Mahomed et al., 2011). The properties of a post-exposure vaccine that primes T cells to the optimal state of differentiation in these endemic regions are probably very different than those for vaccines that achieve this in naive individuals. These considerations are usually overlooked; most TB vaccines are tested pre-clinically in animals that are naive to mycobacteria. Vaccines eliciting T cells that recognize subdominant Mtb antigens and possess TCRs that interact with low avidity to Mtb-infected cells may be less prone to terminal differentiation and promote more durable immunity. Such an approach has shown promise in inbred mice, but may be difficult to achieve in human populations because subdominant antigens will vary between individuals due to HLA diversity.
Overall, our studies demonstrate the value of crosstalk between mouse and human TB research. Currently, human Mtb infection is categorized in a binary fashion into either latent infection or active disease based on immune reactivity (e.g., QFT status) and the presence or absence of clinical symptoms and/or detection of bacilli in sputum. We propose that using FDS of CD4 T cells to measure the relative availability of distinct subsets of Mtb antigens in different strata along the spectrum of Mtb infection may facilitate more precise classifications. For example, FDS determinations may help identify individuals classified by a memory T cell response as having latent Mtb infection, who have actually cleared infection; conversely, individuals with subclinical disease, or active TB disease with a high degree of ongoing Mtb replication, may also be identified. Future studies are needed to determine if this approach could have prognostic value and shape clinical decision-making.
