To download the complete booklet, click here.
OVERVIEW
Early days.
The South African Tuberculosis Vaccine Initiative (SATVI) was first known as the “BCG Study” research group, before the SATVI name was adopted. The research group was located within the Child Health Unit of the University of Cape Town’s Faculty of Health Sciences.
The BCG Randomised Clinical Trial, 2000.
A small group of health scientists associated with UCT and Rockefeller University, including Professors Greg Hussey, Maurice Kibel (deceased) (UCT), Gilla Kaplan and Willem Hanekom (Rockefeller University), conducted studies of TB epidemiology in children, BCG vaccine administration, and the immune response to TB and BCG vaccination. These early studies set the scene for later clinical trials of novel TB vaccine candidates. In South Africa, BCG vaccine (Tokyo 172 strain) was given percutaneously using a multi pronged, BCG-coated administration tool. Vaccination policy in South Africa changed to intradermal administration of BCG (Danish 1331 strain) in 2000.
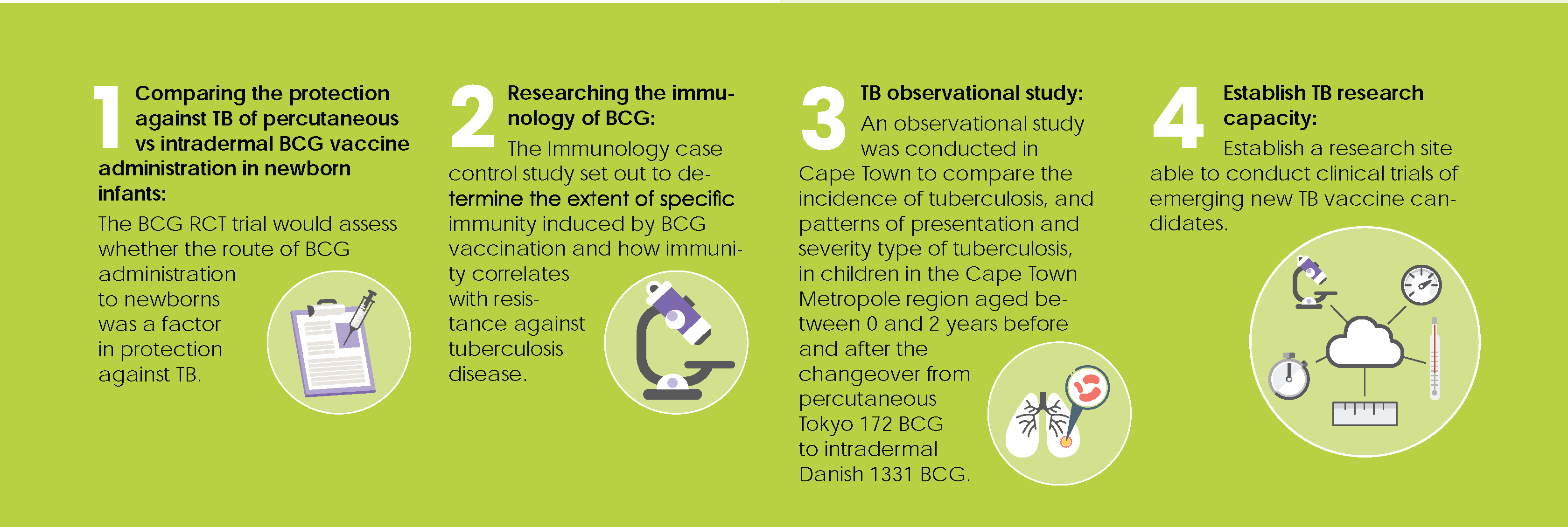
Epidemiological and immunological studies to define the TB epidemic.
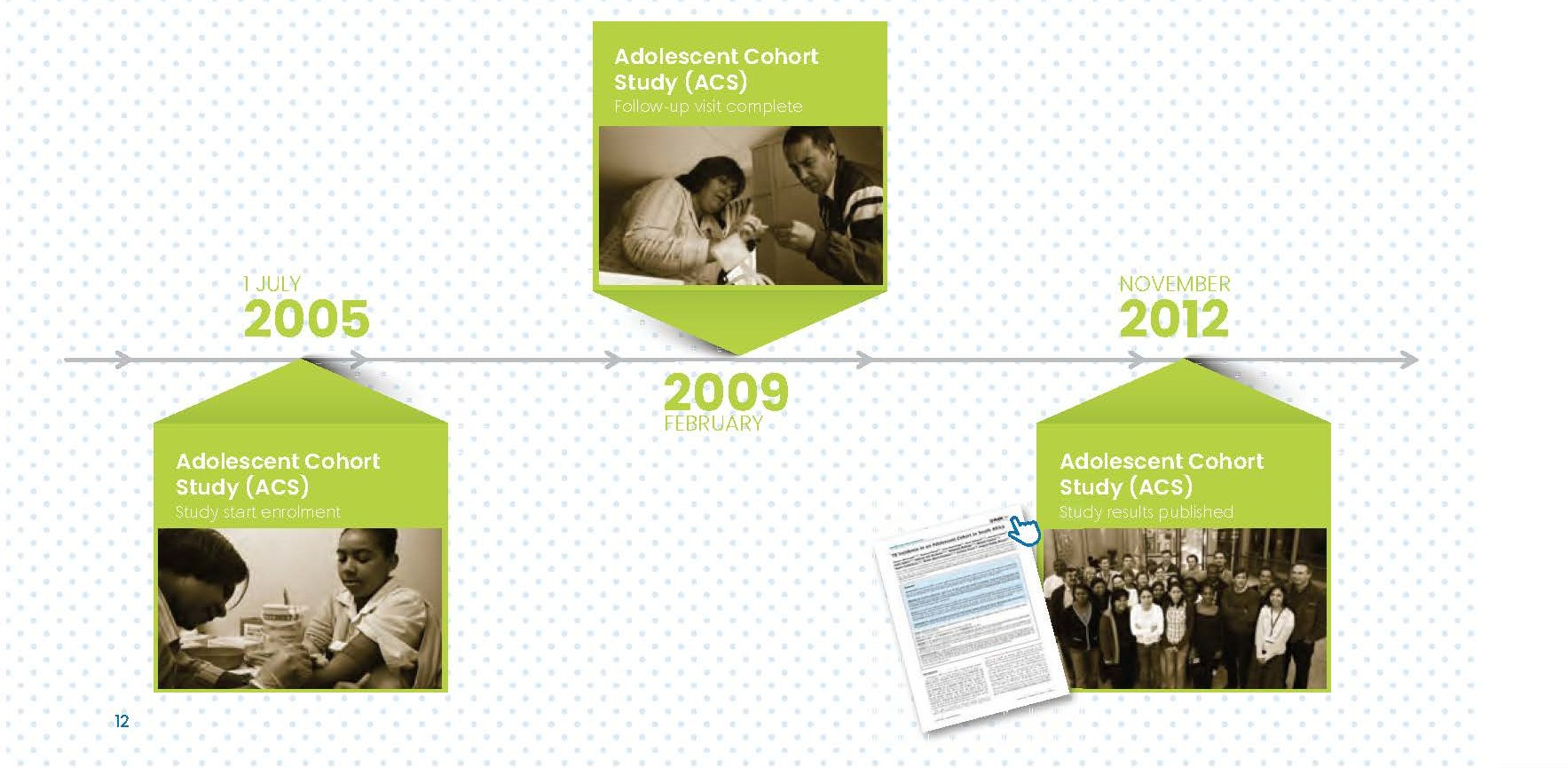
Between 2004 and 2009 SATVI conducted three large-scale epidemiological studies amongst various age groups and subpopulations to generate data about TB prevalence and incidence in this area to lay the basis for late phase TB vaccine studies. These studies were the Adolescent Cohort, the Neonatal Cohort and the Adult Prevalence Study.
MVA 85, the first new generation infant TB vaccine candidate in 50 years.
The period from September 2005 onward marked a major shift in SATVI’s research focus from large epidemiological studies to conducting clinical trials of novel candidates in the growing TB vaccine pipeline.

Multiple studies of TB vaccines, drugs, immunological and diagnostic tools.
In the period 2000 to 2012, SATVI conducted clinical trials of BCG and 5 new TB vaccines in 11 different protocols. These studies included an infant efficacy trial of the viral vectored candidate vaccine MVA85A and early Phase 1-2 trials of Aeras-402 vaccine and the subunit candidate vaccines H4:IC31, M72/AS01E and H56:IC31.
Immunology.
Since its establishment in 2000, SATVI has established two Good Clinical Laboratory Practice compliant immunology laboratories with excellent scientific teams that facilitate the conduct of cutting-edge immunology studies in a rural field-setting at the heart of the TB pandemic.
SATVI has completed many seminal immunology projects that have contributed substantially to advancing global understanding of immune responses to Mycobacterium tuberculosis and to TB vaccination in humans. Much of the early work focused on developing and optimising practical and rigorous methods to SATVI has also established state of the art immunology technologies and relevant expertise to measure and understand the complex interactions between the immune system, Mycobacterium tuberculosis and vaccines.
Building the SATVI team.
Capacity development was a focus from the early days of SATVI, building not only research infrastructure and operating systems, but investing in our people.

Our impact.
SATVI has been extraordinarily productive in the clinical trials arena, having conducted more than a dozen Phase I-IIb trials of seven novel TB vaccine candidates, among more than 3,000 research participants. Additionally, the group has enrolled more than 20,000 participants in observational and immunological TB studies and clinical trials of BCG vaccine. The SATVI postgraduate program has produced many PhD and Masters graduates since 2006. The group has a prolific publication output with a number of high-impact and highly cited papers. To review our publication output, click here.SATVI published the findings of the first efficacy trial of a new infant TB vaccine (MVA85A) in almost 50 years (Tameris, Hatherill et al., Lancet 2013).
SATVI Directors since its establishment in 2001:-
- Professor Greg Hussey
- Associate Professor Tony Hawkridge
- Dr Hassan Mahomed
- Professor Willem Hanekom
- Professor Mark Hatherill
Resource list
Marking 10 years of a history of SATVI, 2012.
The history of SATVI appeared in a the South African Medical Journal (June 2012) in a journal article titled "SATVI – after 10 years closing in on a new and better vaccine to prevent tuberculosis" and SATVI was profiled in The Lancet in 2013 in an article titled "Profile: SATVI a leading light in tuberculosis vaccine research".
Documenting the SATVI history 2021
This SATVI History book is the result of more than 20 interviews with SATVI staff and students, including many colleagues who have left, but are very much still part of the SATVI family. A small volunteer working group of SATVI historians set out to identify key events and to conduct the interviews with role players, a process that created a rich oral history of SATVI for our staff and students, our collaborators and the wider TB research community. We hope that it will inspire the many unsung SATVI TB research heroes to record their own stories, continue the narrative, and build on the incredible institutional memory that sustains the SATVI Mission: Innovative and High Quality TB Vaccine Research in Africa to Impact the Global Epidemic.


