(and a little bit of history)
Design of the protein shell
The complex arrangements of macromolecules in the virus shell are minute marvels of molecular architecture. Specific requirements of each type of virus have resulted in a fascinating apparent diversity of organisation and geometrical design. Nevertheless, there are certain common features and general principles of architecture that apply to all viruses.
In 1956, Crick and Watson proposed on theoretical considerations and on the basis of rather flimsy experimental evidence then available, principles of virus structure that have been amply confirmed and universally accepted.
They first pointed out that the nucleic acid in small virions was probably insufficient to code for more than a few sorts of protein molecules of limited size. The only reasonable way to build a protein shell, therefore, was to use the same type of molecule over and over again, hence their theory of identical subunits.
The second part of their proposal concerned the way in which the subunits must be packed in the protein shell or capsid. On general grounds it was expected that subunits would be packed so as to provide each with an identical environment. This is possible only if they are packed symmetrically. Crick and Watson pointed out that the only way to provide each subunit with an identical environment was by packing them to fit some form of CUBIC SYMMETRY. A body with cubic symmetry possesses a number of axes about which it may be rotated to give a number of identical appearances. These predictions were soon confirmed and it became evident that the occurrence of icosahedral features in quite unrelated viruses was not a matter of chance selection but that icosahedral symmetry is preferred in virus structure.
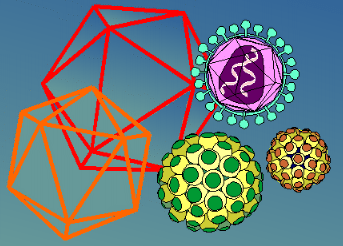
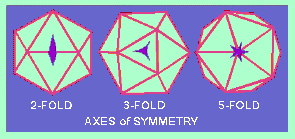
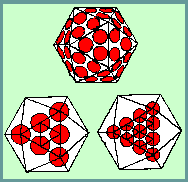
An ICOSAHEDRON is composed of 20 facets, each an equilateral triangle, and 12 vertices, and because of the axes of rotational symmetry is said to have 5:3:2 symmetry
Axes of Symmetry
There are, in fact, six 5-fold axes of symmetry passing through the vertices, ten 3-fold axes extending through each face and fifteen 2-fold axes passing through the edges of an icosahedron.
Icosahedral symmetry requires definite numbers of structure units to complete a shell. In their discussions, Crick and Watson (1956), thinking in terms of asymmetrical protein subunits packed in such a way that each has an identical environment, pointed out that a virus with 5:3:2 symmetry required a multiple of 60 subunits to cover the surface completely. Each unit would be related identically and asymmetrically with its neighbours, and none of the units would coincide with an axis of symmetry.
The introduction of NEGATIVE STAINING (Brenner and Horne, 1959) revolutionized the field of electron microscopy of viruses. Within just a few years, much new and exciting information about the architecture of virus particles was acquired. Not only were the overall shapes of particles revealed but also the symmetrical arrangement of their components. This led to a need for a new terminology to describe the viral components.
Lwoff, Anderson and Jacob (1959) proposed the terms "capsid" and "capsomers" to represent, respectively, the protein shell and the units comprising it, and the term"virion" to denote the complete infective virus particle (i.e. a capsid enclosing the nucleic acid). This terminology was generally accepted although it later proved to be inadequate.
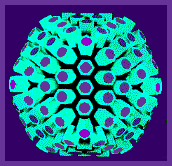
As soon as the first high resolution micrographs of negatively stained icosahedral viruses were obtained (Horne et al., 1959 - adenovirus; and Huxley and Zubay, 1960 - turnip yellow mosaic virus) it seemed that there was a structural paradox. The number of morphological units observed on the surface of known icosahedral viruses at that time was never 60 or multiples of 60, and was often more than 60. Furthermore, the capsomers themselves appeared to be symmetrical and were located on symmetry axes, e.g. herpesvirus.
Below is a model of the herpes simplex virus capsid
There was direct evidence that capsomers of herpesvirus were hexagonal and pentagonal in section. It is obvious that five-fold capsomers must be located on axes of five-fold symmetry, and six-fold capsomers may be situated on axes of two-fold or three-fold symmetry, or in indifferent sites where they are suited to hexagonal packing.
It was therefore clear that the capsomers were not equivalent to the subunits of Crick and Watson (1956).
An obvious solution to the problem was provided by supposing that the symmetrical capsomers are built from a number of ASYMMETRICAL SUBUNITS. In this way it is possible to build a variety of complicated bodies in which 5:3:2 symmetry is preserved and in which the number of subunits is a multiple of 60 as predicted by Crick and Watson.
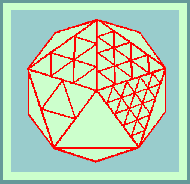
The theoretical basis for the structure of isometric viruses was put on a firm foundation by Caspar and Klug (1962) with their concept of identical elements in quasi-equivalent environments. They defined all possible polyhedra in terms of structure units. The icosahedron itself has 20 equilateral triangular facets and therefore 20T structure units where T is the TRIANGULATION NUMBER given by the rule:
T=Pf
where P can be any number of the series 1,3,7,13,19,21,31 ..(=h
+ hK +K
, for all pairs of integers, h and K having no common factor) and f is any integer.
Morphological units can be clustered as 20T trimers, 30T dimers or separated as 60T monomers. The number of morphological units that would be produced by a clustering into hexamers and pentamers can be calculated as follows: There are 10(T-1) hexamers plus 12. (and only 12) pentamers.
Caspar and Klug (1962) claimed that most icosahedral viruses fall into 2 classes:- P=1 and P=3; and that all deltahedra for which P=>7 are skew, and therefore exist in right and left- handed forms. One "hand" might be selected by the nucleic acid, but there would still be the chance that mistakes in assembly leading to defective particles might occur. The most probable mistake in assembly would be the formation of tubular forms. Tubular structures which have a diameter and surface structure similar to icosahedral virus particles have been observed associated with polyoma and papilloma viruses.
In a review of symmetry in virus architecture, Horne and Wildy (1961) showed that all the viruses then known (with the exception of a few bacteriophages) fell into two main morphological groups -
those with cubic symmetry
and the others with HELICAL symmetry.
"Linear" viral capsids have RNA genomes that are encased in a helix of identical protein subunits.
The length of the helical viral nucleocapsid is determined by the length of the nucleic acid.
Until 1960, the only known examples of virions with helical symmetry were those of plant viruses,
the best studied example being tobacco mosaic virus.
At that time, the architecture of the myxoviruses was poorly understood. Early electron micrographs of shadow-cast preparations revealed particles of varying shape and size but little detail could be reported (Bang, 1948). With the advent of negative staining, it became obvious that the myxo- and paramyxo-viruses consisted of an inner nucleo-protein component with helical symmetry surrounded by an envelope of characteristic morphology. This realization of the helical symmetry of the myxoviruses laid the foundation for the understanding of the symmetry of other complex groups of viruses such as rabies virus and granulosis virus.
In an attempt to clarify the terminology for virus components, Caspar et al. (1962) made a number of proposals which were generally accepted. Briefly, the proposals are as follows:
- The CAPSID denotes the protein shell that encloses the nucleic acid. It is built of structure units.
- STRUCTURE UNITS are the smallest functional equivalent building units of the capsid.
- CAPSOMERS are morphological units seen on the surface of particles and represent clusters of structure units.
- The capsid together with its enclosed nucleic acid is called the NUCLEOCAPSID.
- The nucleocapsid may be invested in an ENVELOPE which may contain material of host cell as well as viral origin.
- The VIRION is the complete infective virus particle
© Copyright Dr Linda M Stannard, 1995
This page was written by Dr Linda Stannard, on behalf of the Division of Medical Virology, UCT.
In Memory of Dr Linda Stannard, 10 May 1942 - 17 October 2016
For re-use of and queries about Dr Stannard's images, please contact Dr Stephen Korsman at stephen.korsman@uct.ac.za.
Recipient of Key Resource Award