CIDRI-Africa enrolls first patient in the MESA-TB study
The CIDRI-Africa team began screening potential participants for MESA-TB at our facility at Site B Community Health Centre in Khayelitsha, Cape Town, on the 29th of March 2021, and were excited to enroll the first patient on the 12th of April.
The trial follows the success of a previous phase 2b study of the M72/AS01E tuberculosis (TB) vaccine, which found that the vaccine reduced the rate of TB lung disease in HIV negative adults by half. MESA-TB goes further, examining whether M72/AS01E is safe and immunogenic in healthy HIV positive people. People living with HIV who are between 18 and 35 years of age, who have been on antiretroviral therapy (ART) for at least 3 months, and who have previously received TB preventive therapy are eligible to join the trial at CIDRI-Africa’s clinic.
"After the disruptions and hardships of the last year it was heartening to get back on track in the quest to advance vaccination against tuberculosis. Hopefully some of the energy devoted to preventing COVID-19 can now be harnessed in the fight against tuberculosis," commented Robert Wilkinson, CIDRI-Africa director.
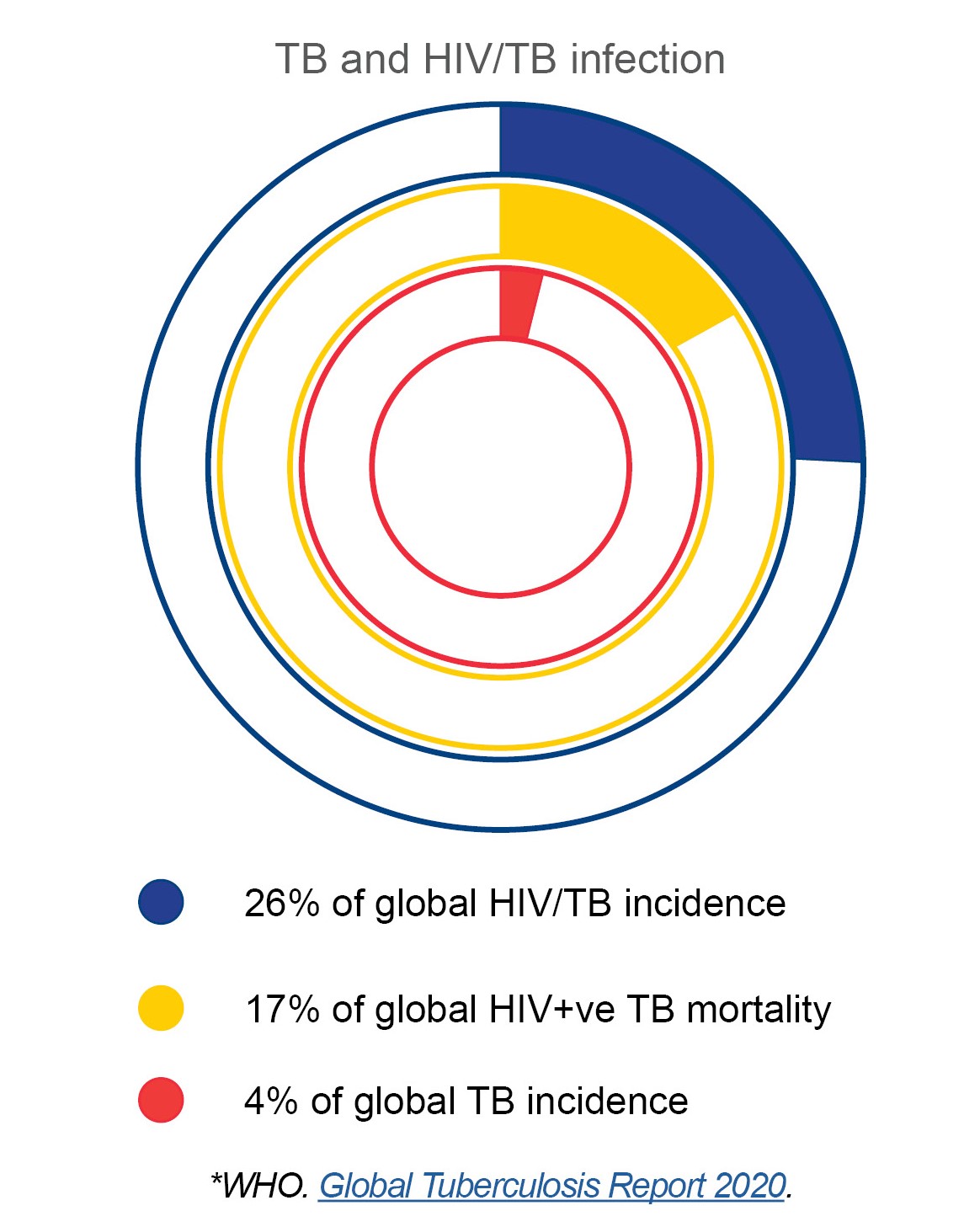
In South Africa, approximately 615 out of every 100 000 people contract TB every year—put another way, an estimated 360 000 South Africans developed TB in 2019. Some 58 000 South Africans died of TB disease that year. TB continues to cause misery as the leading global cause of death from infectious disease, and is particularly serious in countries with a high burden of HIV infection such as South Africa.
Tuberculosis is a leading cause of death among people living with HIV; HIV coinfection is the most important risk factor for developing active TB disease, increasing both the susceptibility to primary infection or reinfection, and the risk of TB reactivation. Antiretroviral therapy is currently the most effective TB prevention strategy for people living with HIV, but even with widely available ART TB incidence is still higher in HIV positive compared with HIV negative South Africans. A TB vaccine effective in both healthy and immunocompromised individuals, including those who are living with HIV, could make all the difference.
The MESA-TB study will help determine whether M72/AS01E fulfills that hope. The trial, fully titled “A randomized, placebo-controlled, observer-blind, phase 2 study to evaluate safety and immunogenicity of the investigational M72/AS01E Mycobacterium tuberculosis (Mtb) vaccine in virally suppressed, antiretroviral-treated participants with human immunodeficiency virus (HIV)”, will take place at six sites around South Africa. Study participants will receive two doses of vaccine or placebo 28 days apart; all participants will be seen at the clinic again within 7 days after each injection to monitor their well-being. They will continue to be followed up for safety, reactogenicity and immunogenicity assessments for one year after the second dose.
MESA-TB is sponsored by the Bill & Melinda Gates Medical Research Institute.
